Abstract
Introduction: Patients with polycythemia vera (PV) have an increased risk of morbidity and mortality from thromboembolic events and cardiovascular disease, particularly for hematocrit (Hct) levels > 45% (Marchioli et al., 2013). Maintaining Hct < 45% is therefore important to reduce these risks. A device that patients can use to frequently monitor their own Hct levels could provide a detailed picture of the change in Hct over time, enable timely clinical visits and disease management, reduce disease burden, and increase patients' quality of life (QoL). A meter to measure hemoglobin (Hb) and Hct that has been approved for medical professional use (StatStrip Xpress ® 2 Hb/Hct meter, Nova Biomedical Corporation) has potential as an at-home, self-testing device.
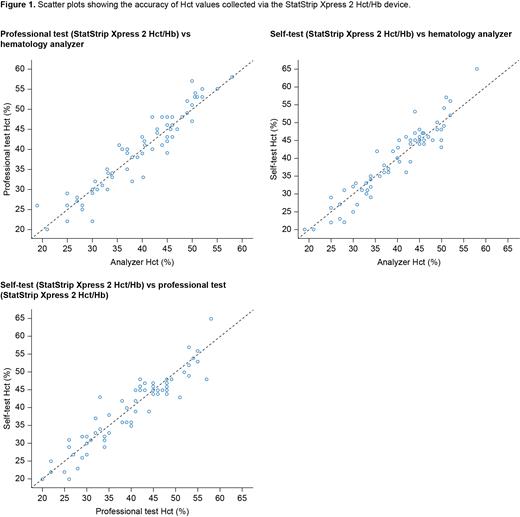
Purpose: To carry out a proof-of-concept study in clinical practice to test the accuracy of the StatStrip Xpress 2 Hb/Hct meter, to evaluate its feasibility for patient self-testing, and to assess its impact on patient-reported outcomes.
Methods: Adults (aged > 18 years) who had PV (according to the World Health Organization 2016 criteria), or conditions with Hct < 35% or Hct > 50%, who required monitoring of Hct/Hb values in clinical practice and who provided informed consent were enrolled from two Swiss centers in this observational study. During each routine visit, patients collected and analyzed a finger-prick blood sample using StatStrip Xpress 2 Hb/Hct (self-test) without guidance or intervention from healthcare professionals (HCPs); HCPs then repeated this test (professional test). At each visit, HCPs also collected and analyzed a venous blood sample using a laboratory hematology analyzer (Sysmex), and patients completed a 15-minute questionnaire about use of the self-test device. The primary endpoint was mean difference in absolute percentage points between Hct values from the professional test and the analyzer (mdiff, 90% confidence interval [CI]; Spearman correlation [r]). Secondary endpoints included mdiff for Hct (%) and Hb (g/dl) between values obtained from the self-test and professional test, and the self-test and analyzer. Other endpoints included the correlation between the professional test Hct values and other blood cell counts. The study was approved by local ethics committees.
Results: Blood measurements from 68 visits for 60 patients (PV = 32; other conditions with Hct < 35% or > 50% = 28) were included in the analysis. For the primary endpoint, Hct values were very similar for the analyzer and professional test (66 data points after removal of 1 outlier; mdiff = 0.1% [CI: ‒0.5-0.8]; r = 0.95, p < 0.001). Importantly, Hct results were also similar for the self-test and professional test (63 data points after removal of 2 outliers; mdiff = -0.2% [CI: ‒0.98-0.5]; r = 0.93, p < 0.001) and the analyzer and self-test (64 data points after removal of 2 outliers; mdiff = 0.1% [CI: ‒0.6-0.8]; r = 0.93, p < 0.001). Similar results were obtained for Hb measurements across the tests. There was no correlation between the accuracy of Hct values from the professional test (vs the analyzer) and blood cell counts, but this should be interpreted with caution because there was a limited number of patients with extreme white blood cell and platelet counts. There was no influence of sex (male/female), age (≤ 75 years/> 75 years) or, in the case of patients with PV, time from diagnosis on the results. Responses to the patient questionnaire showed that 92% of patients were satisfied or very satisfied with the self-test device, 100% found it easy to use, and 97% were willing to start using it at home. Of patients with PV, 71% stated that using a self-testing device would make them feel safer and 56% felt that it would have a positive effect on their QoL.
Conclusions: This study demonstrates the accuracy of the StatStrip Xpress 2 Hct/Hb meter vs a laboratory analyzer for measuring Hct and Hb levels. Importantly, it shows that measurements taken by patients are as accurate as those taken by HCPs. These findings highlight the potential benefits of introducing an instrument for at-home self-testing to the management of patients with PV or other hematological conditions that require regular Hct/Hb monitoring. Benefits to patients may include increased QoL, improved clinical outcomes, and reduced morbidity and mortality through timely clinical action. In addition, fewer outpatient appointments may mean reduced healthcare costs.
Rovo: Novartis: Honoraria; BMS: Honoraria; Amgen: Other: Financial support for congresses and conference travel; AstraZeneca: Other; Swedish Orphan Biovitrum AG: Honoraria; AG Alexion: Honoraria; OrPhaSwiss GmbH: Honoraria; AstraZeneca: Honoraria; BMS: Other; Sanofi: Other; Roche: Other; Novartis: Research Funding; CSL Behring: Research Funding; AG Alexion: Research Funding. Baierlein-Leimbach: Novartis Pharma Schweiz AG: Current Employment. Triemer: Novartis Pharma Schweiz AG: Current Employment. McCarthy-Pontier: Novartis Pharma Schweiz AG: Current Employment. Lehmann: AbbVie: Honoraria; Novartis: Research Funding; Janssen: Honoraria; Swedish Orphan Biovitrum AG: Honoraria; Janssen: Other; Roche: Other; Abbvie: Other: Financial support for congress and conference travel ; Amgen: Other: Financial support for congress and conference travel ; Celgene: Research Funding; Incyte: Honoraria; BMS: Honoraria; Amgen: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal